Regulators Warm Up to Digital Evidence
Medical device makers foresee faster product introduction with the shift to computational modeling.
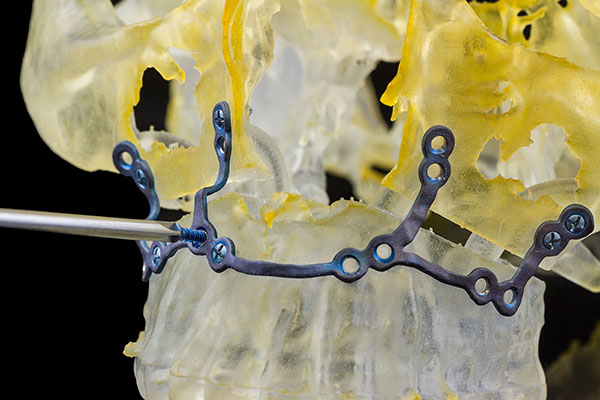
A cranio-maxillofacial implant made possible by Materialise technology. Image courtesy of Materialise.
Latest News
September 8, 2023
The U.S. Food and Drug Administration (FDA) is leading the way in advancement of digital model creation to validate medical devices.
First, in September 2016, the FDA published its guidelines for “Reporting of Computational Modeling Studies in Medical Device Submissions” (FDA-2013-D-1530).
Introducing the document, the agency wrote, “For many years, computational modeling and simulation (CM&S) studies have been used by sponsors to support device design/development and have been reported in medical device submissions … The purpose of this guidance document is to provide recommendations to industry on the formatting, organization and content of reports of CM&S studies.”
The document gave “a nice template for the types of sections you need. This was what we were looking for,” says Wiliam Torres, biomedical engineering and sciences manager, Exponent.
In 2021, during the virtual conference COMSOL Day, hosted by multiphysics simulation software developer COMSOL, Torres gave a keynote on the role of computational models in developing and testing medical implants.
In December 2021, the FDA took the next step. It published a draft guidance document titled “Assessing the Credibility of Computational Modeling and Simulation in Medical Device Submissions” (FDA-2021-D-0980) and began seeking comments from various parties. The comment deadline was March 2022. Leading computational modeling and simulation software developers, including Materialise and Ansys, provided their feedback.
Karen De Leener, cardiovascular market specialist, Materialise, saw these activities as a sign that the FDA is at the forefront in exploring the use of digital models to validate medical devices.

“They understand that it reduces regulatory costs, and also the risk of device failure later on,” she says.
In March 2023, the International Medical Device Regulators Forum (IMDRF), hosted by the European Commission on behalf of the EU, met in Brussels, Belgium. When it meets again in the U.S. in 2024, Thierry Marchal, secretary general at Avicenna Alliance, plans to be there. Marchal also happens to be the chief technologist for healthcare at Ansys.
In this article, we look at how the healthcare regulatory bodies are warming up to digital evidence, how material science is evolving and how the term “digital twins” may mean something different in healthcare.
The Science of Flesh
One tricky aspect of simulating a medical device is replicating its interaction with the patient—or, rather, the patient’s body. Classic simulation solvers employed in aerospace and automotive are proven to be capable of replicating mechanical events. And the characteristics of standard manufacturing materials like steel or aluminum—even newer composite materials—are well understood and well documented.
In many simulation software programs, they’re now readily available as options in a drop-down menu or choices in a dialog box. But the human body with its networks of blood vessels and nerves is a complex biological system of diverse materials, still a mystery in many respects. Therefore, when a stent is placed in a patient’s heart, or an artificial joint replaces a patient’s knee, the material science gets much more complex.
“The moment you take the tissue out of the body, you’ve taken it out of its natural environment, so if you make measurements on it, it’s not a true representation of how live tissues behave,” says Marc Horner, distinguished engineer for healthcare, Ansys. “We’re starting to get a handle on it, but there’s still a lot we don’t know.”
Ansys offers Ansys Discovery, cloud-hosted simulation software targeting the early design phase. Marchal says, “Ansys Discovery uses the Fluent solver. The users know the accuracy of Fluent. It’s accurate enough to give physicians a good guideline. It can produce results quickly, in a matter of seconds, and that’s what the users need to make quick decisions.”
“The starting point is the anatomy of the patient,” says De Leener. “We start with medical imagery, then develop the 3D model with Materialise’s technology assisted by [artificial intelligence (AI)]. Once we have the 3D model, a whole new world opens up.”
In the field of structural heart intervention, the use of 3D-printed true-scale heart models to plan surgical procedures is becoming more common. The ability to rotate and inspect the digital model or printed model in a way that is not possible with a real heart, and even zoom into specific sections to understand various defects, gives clinicians better insights.
“It helps you pick the right device to fit a specific patient, decide the right size and position of the implant,” says De Leener. “Many hospitals are building 3D printing labs in house. It brings 3D medical imagery to a new level.”
Physics of Heat, Fluid and Structure
Since many implants include power sources, heat management is an important design consideration. If the device inside a patient’s body begins to heat up, it could affect nearby muscles and tissues, causing complications.
“If you have any heat-emitting device inside the body, you are dissipating some energy into nearby tissues. Designers have to make sure this does not lead to problems, especially if the device is around your heart. This is where simulation plays a key role,” says Marchal.
Often, medical device design involves more than one type of physics to account for the different phenomena occurring simultaneously. This is the reason Exponent’s Torres relies on COMSOL Multiphysics and other software that allows for the consideration of multiple types of physics that are influenced by one another.
“For [radio frequency]-induced heating inside of an [magnetic resonance imaging (MRI)], you have electromagnetic fields coupled with heat transfer mechanisms. Alternatively, for drug-eluting stents, you have fluid-structure interactions coupled with transport kinetics,” he says.
Politics and Economics of Simulation
Whereas the FDA sets guidelines for in silico tests in the U.S., in Europe it falls under the purview of the European Union Medical Device Regulation (MDR), and in the U.K., under the Medicines and Healthcare Products Regulatory Agency (MHRA). Some believe the work done to incorporate digital evidence into the regulatory pipeline have wider implications, from innovation to talent recruitment.
Marchal points out, “If you can submit digital evidence, that means it is cheaper and faster to submit results and get a product approved without ever compromising patient safety. If the U.S. FDA takes the lead on that, it means the American citizens get access to new innovations faster. Also, many companies might consider relocating to North America because they can get their products approved faster.”
In silico technology and processes are also a disruption to the existing industries and commerce built around medical device testing and manufacturing, so it faces points of resistance.
“It can be cheaper and more accurate to do computational modeling versus benchtop studies or longitudinal clinical studies,” says Torres. “But there’s the risk of the unknown: Will it be accepted by the regulatory agency? So many businesses would rather spend a million dollars for the sake of certainty than to spend $100,000 and accept some uncertainty and the possibility of extended timelines.”
“Acceptance and implementation won’t happen overnight,” says De Leener. “Those digital models and technologies still need validation, not only by the regulatory bodies but also other stakeholders.”
Getting Better Over Time
In the early days of 3D printing, the limited material choices available for printing prevented many medical applications, where the material must be compatible with human biology. But the latest advancements in material science have removed many barriers.
A notable case was that of Kaiba Gionfriddo, a child born with severe tracheobronchomalacia. The treatment involved the use of a 3D-printed bioresorbable splint to restore a collapsed bronchus.
“On February 9, 2012, the specially designed splint was placed in Kaiba at C.S. Mott Children’s Hospital. The splint was sewn around Kaiba’s airway to expand the bronchus and give it a skeleton to aid proper growth. Over about three years, the splint will be reabsorbed by the body,” according to Materialise’s published case study.
Glenn Green, M.D., associate professor of pediatric otolaryngology at the University of Michigan, and his colleague, Scott Hollister, Ph.D., professor of biomedical engineering and mechanical engineering and associate professor of surgery at the University of Michigan, created the custom device based on a computed tomography scan of Kaiba’s trachea. They used Materialise’s Mimics software as part of the design process.
“Implants that can stay inside the body, like personalized hip or cranial implants, are no longer futuristic. They’re becoming standard practice,” says De Leener.
A Different Kind of Digital Twin
Manufacturers often use the term digital twins to refer to the digital replicas of real-world products, such as airplanes and vehicles. In healthcare, too, use of the digital twin is gaining momentum, but the term may mean something different.
“We start with a patient’s anatomy, and that adds a whole new level of confidentiality,” explains De Leener. “In healthcare, digital twins can also be called virtual patients, as the digital 3D models originate from CT scans or MRI scans of a physical patient.”
In risky operations involving specific patients, the digital twin is usually the digital replica of the target patient. But in early-stage development of treatments, pharmaceutical and medical device firms routinely use digital twins representing the target population (for example, average North American male, age 35 to 50) to create solutions that address a large market. In studying the long-term effects of an implant on patients, for example, “the cost-benefit ratio pushes us towards using a representative human body model average,” says Torres.
But true digital twins also require a steady stream of real-time data to help synchronize the real-world products to its digital counterparts. In healthcare, wearables function as edge devices providing data streams that give constant updates. Medtronic, a medical device company and an Ansys client, is a good example.
“Medtronic CareLink is a web-based platform that provides a window into the patient’s current daily status, from insulin status to their blood glucose levels,” says Horner.
“If you have a pacemaker, the first thing the doctor would do when you go see him or her, is to download the information from it. In fact, your doctor might have received a notice from the pacemaker that you need to go see him or her before you even know about it,” Marchal says.
Zimmer Biomet offers smart knee implants equipped with sensors that capture the wearer’s range of motion, stride length, walking speed and step counts. Horner and Marchal agree that while these systems are not yet being used as digital twins, this level of connectivity and information about individual patients addresses a key requirement for developing patient digital twins.
Understanding Medical Digital Twins
The reliability of a digital model is rooted in the parameters used to construct the model. A digital model based on the characteristics of an average North American male, age 35 to 50, should not be used to make predictions about a patient outside the scope of the model.
One primary difference between manufacturing and healthcare is, “There’s no culture of digital simulation [in healthcare]. Therefore, they don’t know the limitations of simulation,” says Marchal.
In manufacturing, digital twins allow manufacturers to reduce the number of costly and time-consuming physical tests, but they’re not meant to completely replace the physical prototypes. The same can be said of the use of simulation in healthcare.
“In silico has already reduced the need for cadaver labs and animal tests, but it cannot replace them altogether,” clarifies De Leener. “Before you start simulating, it’s important to know the question you want to ask [or] the clinical insight you need. Otherwise, you can get lots of data, but what can you get out of it?”
More Ansys Coverage
More COMSOL Coverage
More Materialise Coverage
Subscribe to our FREE magazine, FREE email newsletters or both!
Latest News
About the Author
Kenneth Wong is Digital Engineering’s resident blogger and senior editor. Email him at [email protected] or share your thoughts on this article at digitaleng.news/facebook.
Follow DE