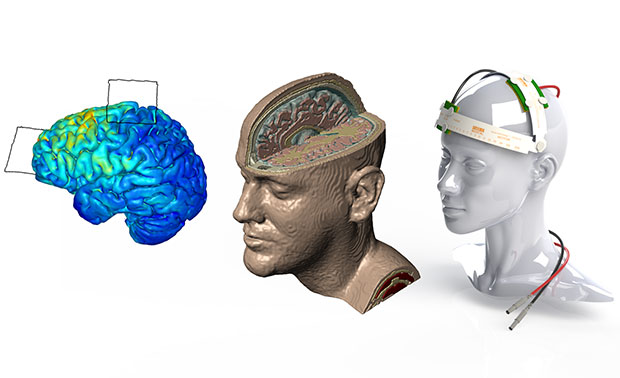
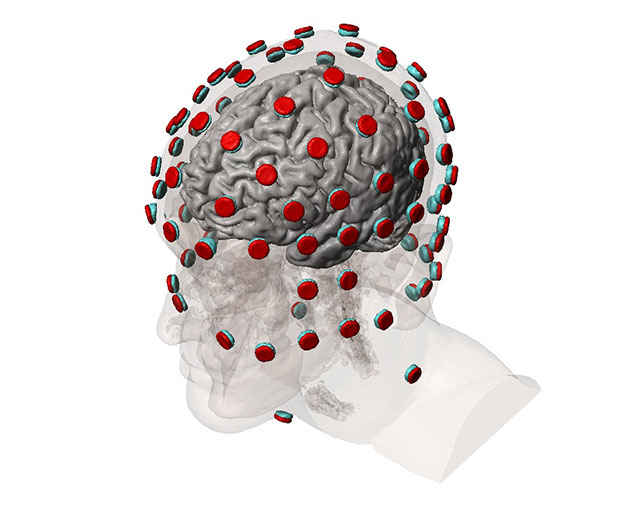
Different stages of brain and head model with tDCS device are shown. Image courtesy of Simpleware.
Latest News
August 1, 2015
Image-based simulation is becoming increasingly common in medical device design, particularly when creating models from 3D scan data such as magnetic resonance imaging (MRI) and computed tomography (CT). Models can be used to evaluate device designs using anatomical data, and can contribute to long-term design decisions and protocols.
Soterix Medical manufactures devices and develops treatments for transcranial direct current stimulation (tDCS), a promising new method for treating neurological and psychiatric disorders through transmission of a low-level direct current (DC) current across the scalp. The New York-based company, in conjunction with the Neural Engineering Group at City College of New York (CCNY), is applying results from Simpleware software to design protocols for tDCS devices. Image-based modeling techniques are used to evaluate performance and reliability.
The Neural Engineering Group, led by Dr. Marom Bikson, focuses on prototyping and verifying tDCS devices. When developing tDCS methods, they needed to investigate the relationship between patient scan data and CAD models of tDCS electrodes through visualization, analysis and export of finite element models, with data used by Soterix Medical for device design.
Another challenge for designing tDCS protocols is to be able to easily customize models to different types of electrode placement montages and current distribution, as well as for various kinds of pathologies and material properties within the brain derived from patient data. Models therefore have to be both high quality enough to reproduce the complex internal and external features of the brain, and able to be modified to suit different protocols.
Simpleware software was chosen as the solution for generating and modifying finite element models of the human head from MRI data, with COMSOL Multiphysics used as the solver for applying electrostatic volume conductor and other physics. This approach has allowed Soterix and CCNY to develop detailed head models to optimize device designs to specific conditions and clinical targets.
Simulating Device Designs
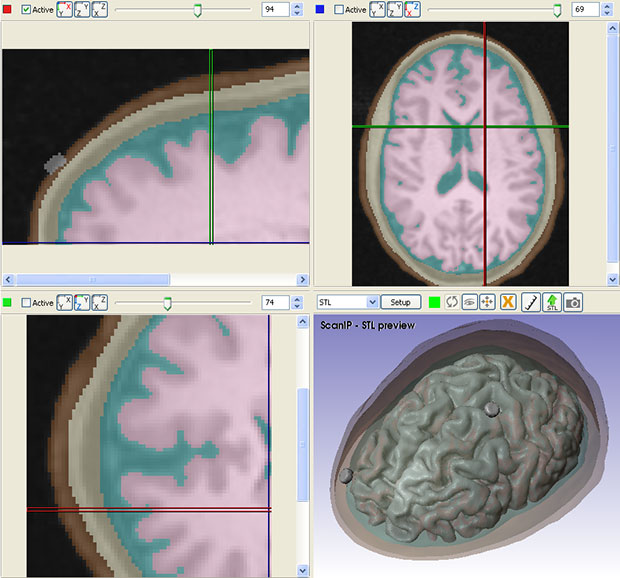
A standard workflow for simulating tDCS device performance involves obtaining 3D image data from an MRI procedure. Researchers can then use in-house image segmentation algorithms to define different parts of the brain and skull. Simpleware software is then employed for manual segmentation of image data, particularly for demarcating between tissue compartments. In addition, researchers use Simpleware software to import and place CAD parts, such as electrodes, anodes, cathodes and sponge modes into segmented image data in specific configurations.
From this segmented image data, researchers can generate multiple finite element models, or meshes, for simulation. Simpleware software’s capabilities for directly generating volume meshes from image data, rather than meshing from CAD geometries in a solver, means that researchers at CCNY are able to rapidly obtain different models. Refinements can be made to meshes to specify element sizes and work with computing resources. The accuracy of these models is crucial for simulating electrical currents in COMSOL Multiphysics. Electrostatic volume conductor physics are then applied in the solver, with exterior boundaries and interior boundaries defined. Anode placement is also defined to take into account different types of current flow, and material properties assigned depending on the simulation.
Assisting Clinical Trials
Results from simulations have been used towards designing clinical trials and informing thinking on areas such as dosage levels. Work is being carried out towards understanding tDCS for schizophrenia, cognitive multi-tasking, stroke rehabilitation and pediatric cases. Dr. Marom Bikson notes: “It’s critical to precisely simulate the interaction of technology with biology for the purpose of optimization design prior to clinical testing” with computational modeling “based heavily around Simpleware software” allowing a wide range of analyses to be carried out.
“Soterix Medical Inc. has adapted such designs for devices in clinical trials in over 200 medical centers, meaning thousands of patients are enrolled in clinical trials with devices designed using Simpleware,” says Bikson. “The success of these trials is linked to the quality and flexibility of Simpleware software.”
More Info:
Subscribe to our FREE magazine, FREE email newsletters or both!
Latest News
About the Author
DE’s editors contribute news and new product announcements to Digital Engineering.
Press releases may be sent to them via [email protected].